Global Health
Convalescent plasma therapy – is it an efficient approach to treating COVID-19?
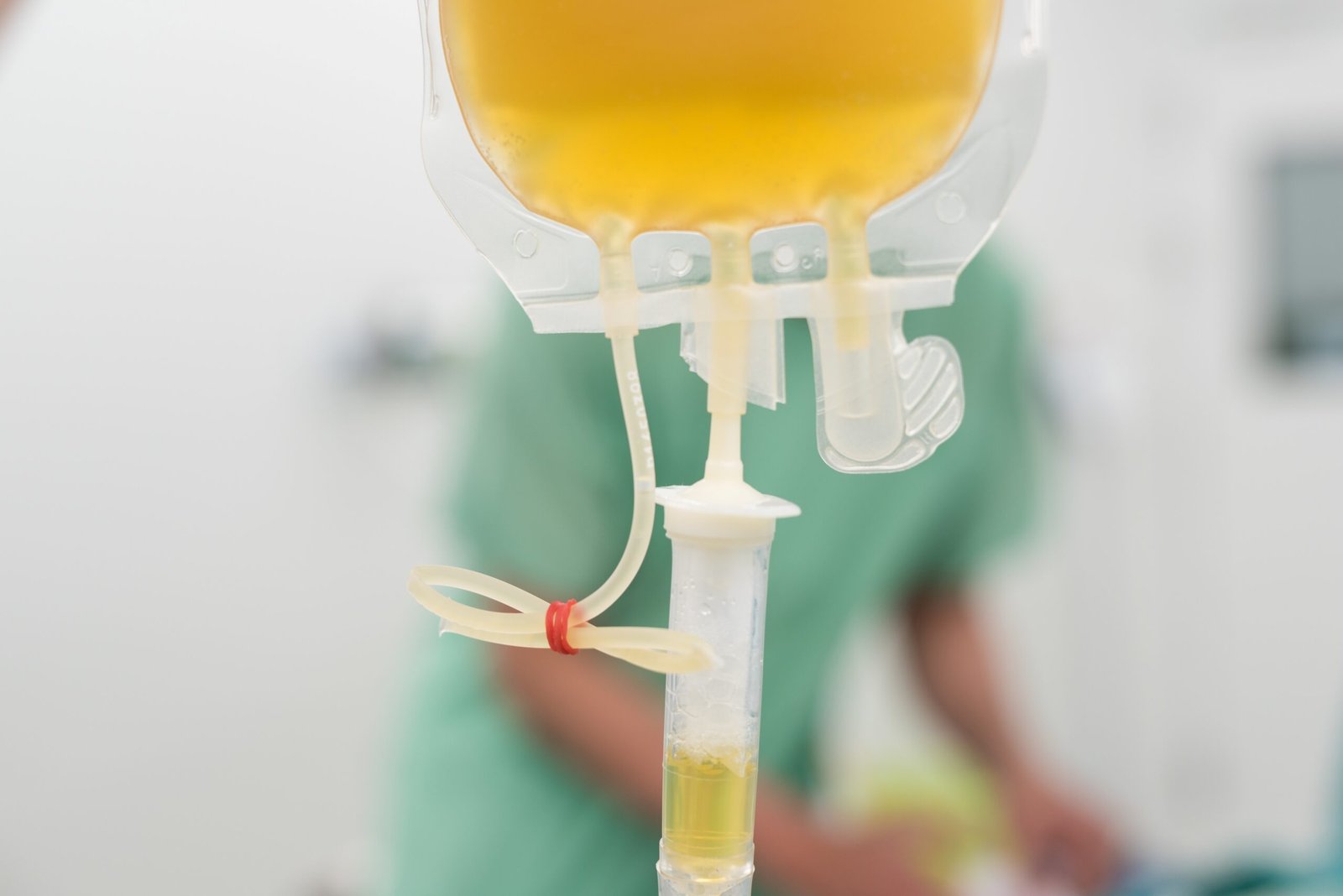
There are promising signs that the COVID-19 curve is starting to flatten, however the pandemic is much from over. Scientists are working hard to seek out a preventive vaccine and to prove the efficacy of many currently available drugs, including antivirals, antimalarials, interleukin inhibitors, and protease inhibitors. Convalescent plasma (CP) is one other therapeutic strategy being studied to treat patients with severe COVID-19.
The use of convalescent blood products (CBP) within the treatment of infectious diseases dates back to the late nineteenth century, after they were first used to treat diphtheria. Since then, CBP has been used to treat bacterial infections reminiscent of scarlet fever and whooping cough (Marano et al., 2016). The therapeutic regimen was studied in the course of the Spanish flu pandemic of 1918–1920 and later as a treatment for measles, Argentine hemorrhagic fever, influenza, varicella, cytomegalovirus, parvovirus B19, Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), H1N1 and H5N1 avian influenza, and severe acute respiratory syndrome (SARI) viruses (Marano et al., 2016). In 2009, studies of H1N1 influenza showed a discount in mortality in those treated with convalescent plasma, in addition to a discount in viral load inside five days of symptom onset, without serious adversarial events (Chen et al., 2020). CP is currently being studied for the treatment of Ebola in several countries, nevertheless, final results are awaited after complete data collection. Most studies of CP lack randomization, control subjects, and enormous sample sizes, due to this fact efficacy has not been proven but is as an alternative considered empirical or anecdotal.
CP therapy is predicated on the concept of acquired passive immunity, which develops after an individual receives immune components, reminiscent of antibodies, from one other person. This kind of immunity provides immediate protection against an antigen, but it surely is just not long-lasting. CP, which is obtained from a patient who has survived a previous infection and developed humoral immunity against the pathogen, incorporates a considerable amount of neutralizing antibodies which can be capable of eliminate the virus from the blood and tissues.
CP is obtained from a donor through apheresis, a process during which whole blood is withdrawn from the donor through veins and passed through a machine during which the blood is separated by filtration or centrifugation into components reminiscent of platelets, plasma, leukocytes, lymphocytes, and red blood cells. Once separated, the specified component of the blood, on this case the plasma, is removed, and the remaining blood is reinfused into the patient. This process takes several hours, much like donating blood.
People who’ve been infected with SARS-CoV-2, the virus that causes COVID-19, and have recovered now have antibodies against the virus of their blood. Antibody levels will decline over time after the initial illness, inside three to 4 months, so plasma from recently recovered patients could also be handiest. US Food and Drug Administration (FDA) issued guidance for the administration and study of experimental convalescent plasma (FDA, 2020).
CP will be collected from individuals meeting the next criteria (FDA, 2020):
- Diagnostic test (e.g. nasopharyngeal swab) on the time of illness, positive serological test for antibodies against SARS-CoV-2 after recovery, if no diagnostic test was performed previously
- One of the next options:
- Complete resolution of symptoms at the least 28 days before donating blood
- Complete resolution of symptoms for at the least 14 days prior to blood donation, negative COVID-19 test results from a number of nasopharyngeal swabs or diagnostic blood tests
- Negative test result for antibodies against human leukocyte antigen (HLA)
- SARS-CoV-2 neutralizing antibody titers, if available
- Neutralizing antibody titers of at the least 1:160 are really useful; a titer of 1:80 could also be considered if another matched unit is just not available
The donated CP should undergo routine laboratory tests:
- Blood group: ABO and Rhesus D (RhD)
- Blood screening for HIV, HBV, HCV, syphilis and other locally transmitted infections
Per FDA guidance (2020), CP may only be administered: 1. In the context of an FDA-approved clinical trial; 2. Under an expanded access protocol for patients with serious or immediately life-threatening COVID-19 disease who’re ineligible or unable to take part in a clinical trial; and three. At the request of a licensed physician within the event of a single-patient emergency.
To receive CP, patients must meet the next criteria (FDA, 2020):
- Laboratory confirmed COVID-19
- Severe or immediately life-threatening course of COVID-19:
- Severe illness is defined because the occurrence of a number of of the next symptoms:
- Dyspnea (shortness of breath)
- Respiratory rate ≥ 30/min
- Oxygen saturation ≤ 93%
- Ratio of partial pressure of arterial oxygen to fraction of inspired oxygen < 300
- Pulmonary infiltrates > 50% inside 24 to 48 hours
- Life-threatening illness defined as a number of of the next:
- Respiratory failure
- Septic shock
- Dysfunction or failure of multiple organs
- Severe illness is defined because the occurrence of a number of of the next symptoms:
Nurses administering convalescent plasma should follow standard precautions for blood administration as outlined of their hospital protocols and punctiliously monitor their patients for potential transfusion reactions.
Finding suitable donors and implementing tests to substantiate appropriate antibody levels in plasma are logistical challenges. In addition to transfusion reactions, one other potential complication of CP therapy is the transmission of unknown pathogens. There can also be a risk of infection for laboratory personnel who process plasma.
Will convalescent plasma play a key role within the treatment and recovery of critically ailing COVID-19 patients? We don’t know, and we’ll should wait until scientists have enough data to properly analyze its effectiveness. Do you have got experience with convalescent plasma therapy? What have you ever observed in your clinical practice? Share it within the comments below.
Chen, L., Xiong, J., Bao, L., and Shi, Y. (2020). Convalescent plasma as a possible therapy for COVID-19. The Lancet. Retrieved from https://www.thelancet.com/article/S1473-3099(20)30141-9/full text
Kim, AY and Gandhi, RT (2020). Coronavirus disease 2019 (COVID-19): Treatment in adults. UpToDate. Retrieved from https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-management-in-adults
Marano, G., Vaglio, S., Pupella, S., Facco, G., Catalano, L., Liumbruno, G. M. & Grazzini, G. (2016). Convalescent plasma: recent evidence for an old therapeutic tool? . Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4781783/
U.S. Food and Drug Administration (2020). Recommendations for Investigational COVID-19 Convalescent Plasma. Downloaded from https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma
-

 Well-Being1 year ago
Well-Being1 year ago5 books that may help at work at work
-

 Global Health1 year ago
Global Health1 year agoThe Global Fund opens up the potential of private sector investment – updates
-

 Well-Being1 year ago
Well-Being1 year agoFast and healthy advice on preparing meals for busy nurses
-

 Well-Being12 months ago
Well-Being12 months agoMaintenance of the nursing engine – each day nurse
-

 Best Practice10 months ago
Best Practice10 months agoSafety within the workplace as an ethical imperative in nursing
-

 Best Practice1 year ago
Best Practice1 year agoA cultural approach to the treatment of neonatal pain
-
 Well-Being12 months ago
Well-Being12 months agoHow to get the standard of sleep for higher mental health
-

 Education11 months ago
Education11 months agoAI for teachers – Nursing Education Network






