Global Health
Alteplase – how does it work?
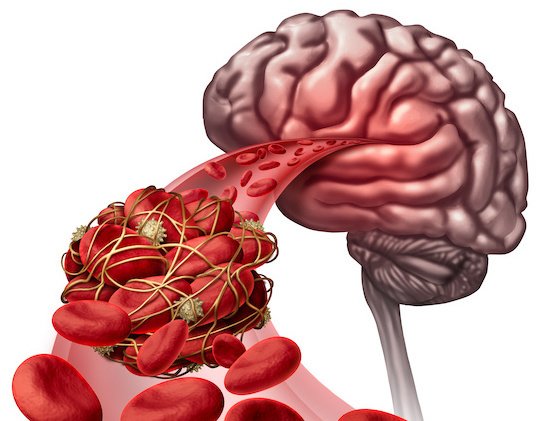
Thrombolytic drugs are commonly utilized in emergency situations to interrupt up or dissolve blood clots which have caused ischemia in essential organs. Alteplase, also often called tissue plasminogen activator (tPA), is a thrombolytic agent produced by recombinant DNA technology. There are two brand formulas approved by the U.S. Food and Drug Administration. The first of those, Activase, is indicated for the treatment of acute ischemic stroke (AIS), acute myocardial infarction (AMI) and acute massive pulmonary embolism (PE). The second, Cathflo Activase, is indicated for unclogging clogged central venous catheters and restoring the function of central venous access devices (Facts and Comparisons, 2022).
How it’s working?
When a clot forms in an artery, it obstructs blood flow, causing ischemia and tissue necrosis. Intravenously (IV) alteplase binds to the fibrin-plasminogen complex within the thrombus and converts inactive plasminogen to lively plasmin, which digests fibrin. Alteplase can dissolve a clot in a coronary or pulmonary artery, restoring blood flow to the world outside the blockage. The initial half-life of alteplase is lower than 5 minutes and it’s primarily cleared by the liver (Genentech, 2018).
.png.aspx?width=650&height=522)
Contraindications (Genentech, 2018)
Alteplase is contraindicated in patients with lively internal bleeding, recent (inside 3 months) severe head trauma or intracranial/spinal surgery, intracranial disease which will increase the danger of bleeding (i.e. intracranial malignancy, arteriovenous malformation or aneurysm), bleeding diathesis , and the presence of severe, uncontrolled hypertension. Additional contraindications in patients after acute ischemic stroke include the presence of intracranial hemorrhage and subarachnoid hemorrhage. Alteplase shouldn’t be administered to patients with acute myocardial infarction or pulmonary embolism if the patient has recently had a stroke.
Monitoring (Genentech, 2018)
Patients receiving alteplase needs to be closely monitored for bleeding and hypersensitivity reactions. Assess the patient’s neurological status and blood pressure usually in accordance with institutional policy and perform laboratory tests reminiscent of hemoglobin, hematocrit, platelets, fibrinogen, and activated partial thromboplastin time.
AND
Full information and dosing could be present in the leaflet that comes with each medicine or within the Nursing2022 Medicines Manual + medicines updates. For useful information, try our NursingCenter Pocket Card: Alteplase Injection for Acute Ischemic Events.
AND
AND
Facts and Comparisons (2022, May 26). Alteplase injection. . https://fco.factsandcomparisons.com/lco/action/doc/retrieve/docid/fc_dfc/5548433
AND
Genetech. (2018, February). Activate prescribing information. Downloaded from Activase.com: http://www.gene.com/download/pdf/activase_prescribing.pdf
-

 Well-Being1 year ago
Well-Being1 year ago5 books that may help at work at work
-

 Global Health1 year ago
Global Health1 year agoThe Global Fund opens up the potential of private sector investment – updates
-

 Well-Being1 year ago
Well-Being1 year agoFast and healthy advice on preparing meals for busy nurses
-

 Well-Being12 months ago
Well-Being12 months agoMaintenance of the nursing engine – each day nurse
-

 Best Practice10 months ago
Best Practice10 months agoSafety within the workplace as an ethical imperative in nursing
-

 Best Practice1 year ago
Best Practice1 year agoA cultural approach to the treatment of neonatal pain
-
 Well-Being12 months ago
Well-Being12 months agoHow to get the standard of sleep for higher mental health
-

 Education11 months ago
Education11 months agoAI for teachers – Nursing Education Network






